Web as a group, play level 1 of the balancing equation game. Pb(oh)2 + 2 hcl æ 2 h2o + 1 pbcl2. 1 n 2 + 3 h 2 2 nh 3; Web there are any references about chemistry word equations worksheet answer key in bellapetra.my.id. ____ h2 + ____ o2 æ ____ h2o.
2 h2 + 1 o2 æ 2 h2o. (d) 1 c4h6o3 + 1 h2o − −→ 2 c2h4o2. N2 + 3 h2 æ 2 nh3. 2 h2 + 1 o2 æ 2 h2o.
Write down the strategies your group uses to balance chemical equations. Kclo3 æ 2 kcl + 3 o2. Double displacement type of reaction:
The answer key is also available in pdf format or if you’d prefer a quick look, an image of the completed sheet can be found here. Using this balancing chemical equations worksheet. 2 fe+ 3 cl2 − −→ 2 fecl3. N2 + 3 h2 æ 2 nh3. N2 + 3 h2 æ 2 nh3.
2 nacl + 1 f2 æ 2 naf + 1 cl2. 2 h 2 + 1 o 2 2 h 2 o; ____ n2 + ____ h2 æ ____ nh3.
Ch4 + 2 O2 Æ 1 Co2 + 2 H2O.
Each question presents an unbalanced equation. 2 h2 + 1 o2 æ 2 h2o. The worksheet prints on a standard sheet of printer paper. Web as a group, play level 1 of the balancing equation game.
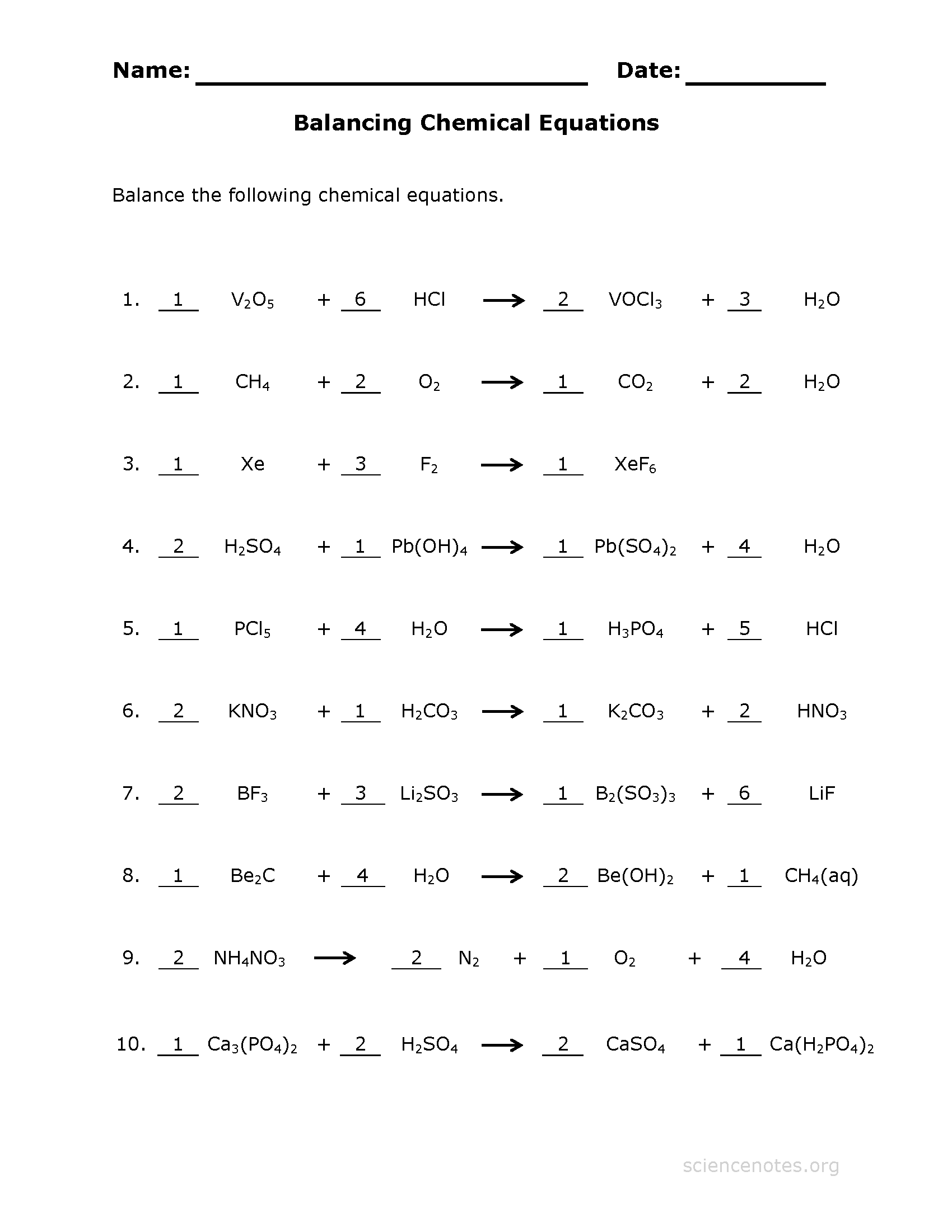
Web Balancing Chemical Equations (Key) Front Side Please Note That Several Of These Equations Are Already Balanced As Written.
Src12+ agn03+ sr(no) + 32 aga pb(no ) 32 type of reaction: The answer key is also available in pdf format or if you’d prefer a quick look, an image of the completed sheet can be found here. Albr3 + 3 k2so4 æ 6 kbr + 1 al2(so4)3. 2 nacl + 1 f 2 2 naf + 1 cl 2;
Feel Free To Download Our Free Worksheets With Answers For Your Practice.
1) 1 n 2 + 3 h 2 2 nh 3 2) 2 kclo 3 2 kcl + 3 o 2 3) 2 nacl + 1 f 2 2 naf + 1 cl 2 4) 2 h 2 + 1 o 2 2 h 2 o 5) 1 pb(oh) 2 + 2 hcl 2 h 2 o + 1 pbcl 2 6) 2 albr 3 + 3 k 2 so 4 6 kbr + 1 al 2 (so 4) 3 7) 1 ch 4 + 2 o 2 1 co 2 + 2 h 2 o 8) 1 c 3 h 8 + 5 o 2 3 co 2 + 4. Please remember that this article is for reference purposes only. Ch4 + 2 o2 æ 1 co2 + 2 h2o. Web balancing chemical equations worksheet.
Every Teacher Is On The Lookout For A Great Worksheet That Can Be Used In Class, Or At Home.
N2 + 3 h2 æ 2 nh3. Pb(oh)2 + 2 hcl æ 2 h2o + 1 pbcl2. 1 pb(oh) 2 + 2 hcl 2 h 2 o + 1 pbcl 2; Kclo3 æ 2 kcl + 3 o2.
Single displacement type of reaction:. 4 fe+ 3 o2 − − → 2 fe2o3. Then i see what elements still need evening out and continue to go back The answer key is also available in pdf format or if you’d prefer a quick look, an image of the completed sheet can be found here. ____ n2 + ____ h2 æ ____ nh3.

![49 Balancing Chemical Equations Worksheets [with Answers]](https://i2.wp.com/templatelab.com/wp-content/uploads/2017/01/balancing-equations-47.jpg)
![49 Balancing Chemical Equations Worksheets [with Answers]](https://i2.wp.com/templatelab.com/wp-content/uploads/2017/01/balancing-equations-11.jpg)