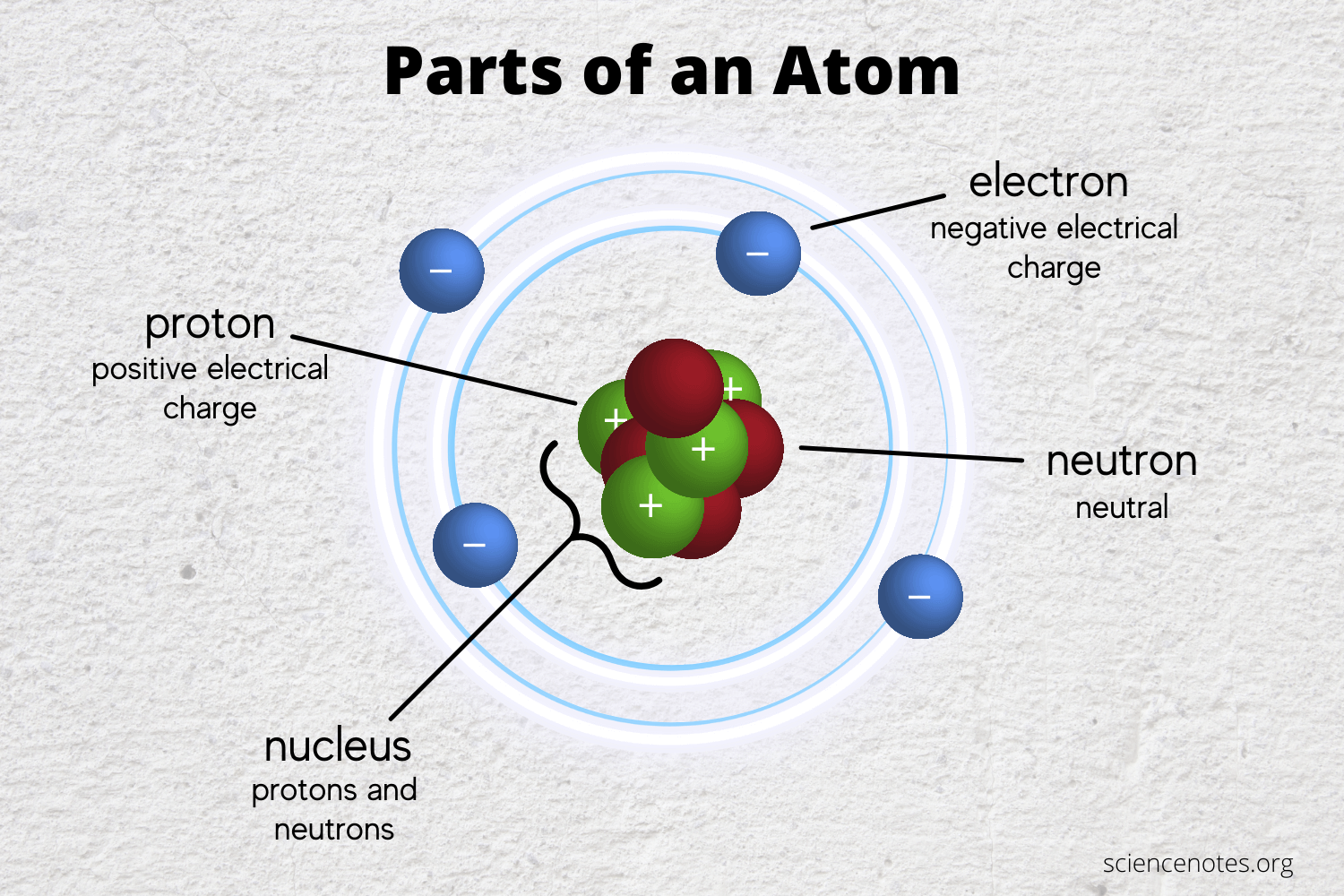
Web a chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. Web the shell closest to the nucleus, 1n, can hold two electrons, while the next shell, 2n, can hold eight, and the third shell, 3n, can hold up to eighteen. A chemical bond is a mutual electrical attraction between the nuclei in valence, electrons of different atoms that binds the atoms. Web a mutual electrical attraction between the nuclei and valance electrons of different atoms that binds the atoms together. Web a chemical bond is the force that holds atoms together in chemical compounds.
Web in general, the loss of an electron by one atom and gain of an electron by another atom must happen at the same time: In order for a sodium atom to lose an electron, it needs to. Web determine whether each of the following bonds would be polar or nonpolar: A molecule is a neutral group of atoms that are held together by covalent bonds.
Web how do atoms make compounds? A chemical bond is a mutual electrical attraction between the nuclei in valence, electrons of different atoms that binds the atoms. Learn about ionic bonds, covalent bonds, polyatomic ions, and metallic bonds, and how they lead to the.
Web in general what determines whether atoms will form chemical bonds? The bond may result from the electrostatic force between oppositely. A molecule is a neutral group of atoms that are held together by covalent bonds. A chemical bond is a mutual electrical attraction between the nuclei in valence, electrons of different atoms that binds the atoms. Web why do some atoms join together to form molecules, but others do not?
(1) covalent bonding, in which. Web a mutual electrical attraction between the nuclei and valance electrons of different atoms that binds the atoms together. Web the shell closest to the nucleus, 1n, can hold two electrons, while the next shell, 2n, can hold eight, and the third shell, 3n, can hold up to eighteen.
Why Is The Co 2 Molecule Linear Whereas H 2 O Is Bent?
The electron arrangement of the outer energy level of an atom determines whether or not. Web a chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. Web in general, the loss of an electron by one atom and gain of an electron by another atom must happen at the same time: A molecule is a neutral group of atoms that are held together by covalent bonds.
Web How Do Atoms Make Compounds?
Web determine whether each of the following bonds would be polar or nonpolar: A chemical bond is a mutual electrical attraction between the nuclei in valence, electrons of different atoms that binds the atoms. Web chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood theory that would not. What determines whether atoms will form chemical bonds is to maximize the stability.
Learn About Ionic Bonds, Covalent Bonds, Polyatomic Ions, And Metallic Bonds, And How They Lead To The.
Web when atoms bond together, they create molecules: Typically they join together in such a way that they lose their identities as elements and adopt a new identity as a compound. Web a chemical bond is the force that holds atoms together in chemical compounds. The bond may result from the electrostatic force between oppositely.
Web The Shell Closest To The Nucleus, 1N, Can Hold Two Electrons, While The Next Shell, 2N, Can Hold Eight, And The Third Shell, 3N, Can Hold Up To Eighteen.
Web discover how atoms and ions come together through chemical bonding! Web the number and arrangement of electrons of an atom determine the kinds of chemical bonds that it forms and how it reacts with other atoms to form molecules. The bond is caused by the. Web a mutual electrical attraction between the nuclei and valance electrons of different atoms that binds the atoms together.
A sodium atom bonds with a chlorine atom to create salt (sodium chloride), two hydrogen atoms bond with an. Chemical bonds are formed when electrons in different atoms. Web a chemical bond is the force that holds atoms together in chemical compounds. The bond is caused by the. The bond may result from the electrostatic force between oppositely.