Explain, in terms of subatomic particles, what is meant by the term isotopes. So different isotopes have different mass numbers but the same proton number. It includes a series of questions of increasing challenge, with answers and extra supporting videos available at the link on the bottom of each page or via the qr code. 6 protons & 6 neutrons e. These isotopes are neutral (charge = 0).
The number 6 refers to the atomic number. Isobars are nuclides of different elements (different \(z\)) with the same mass number (\(a\)). Protons and neutrons are in the nucleus. How many protons and neutrons are in the first isotope?
\textcolor {f21cc2} {\text {number of neutrons}=\text {mass number. Web atoms of the same element with different numbers of neutrons are called isotopes. How many protons and neutrons are in the first.
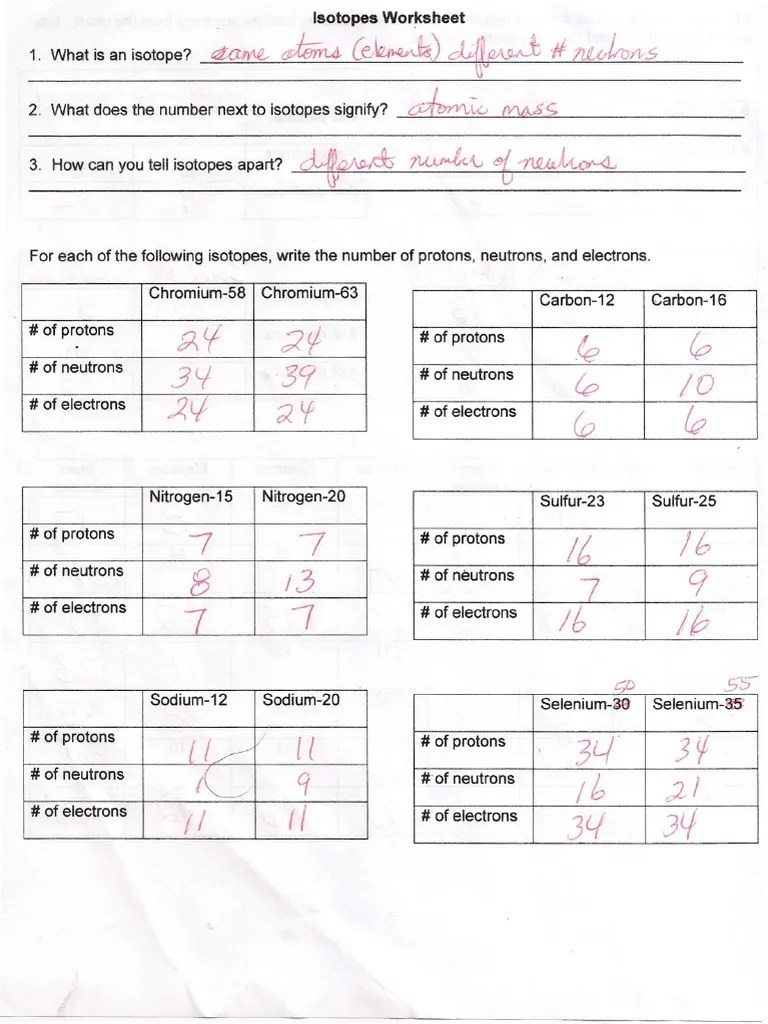
Describe the general arrangement of subatomic particles in the atom electrons surround the nucleus; Web isotopes practice set oe aa — 1. One isotope has a mass number of 10 and the other isotope has a mass number of 11. How can you tell isotopes apart? Web isotopes of an element have the same atomic number (z) but have different mass numbers (a), because they have different numbers of neutrons.
Web pdf, 1.36 mb. How many protons and neutrons are in the first. 32 protons, 38 neutrons, 32 electrons.
(I) 4019 P (Ii) 12050 Sn (Iii) 2963 Cu (Iv) 10947 Au (V) 5826 Fe.
Fill in the following table. Identify which among the isotope symbols below is incorrect. The numbers 12, 13, and 14 refer to the mass number. 12c 6 13c 6 14c 6.
A Short Worksheet To Introduce Or Revise Isotopes.
Web ask the class to read through the remaining sections on the provided worksheet, and answer the questions. How can you tell isotopes apart? The lesson on “atomic structure and notation” is provided free in my other. Web in a sample of e there are two isotopes.
It Includes A Series Of Questions Of Increasing Challenge, With Answers And Extra Supporting Videos Available At The Link On The Bottom Of Each Page Or Via The Qr Code.
What does the number next to isotopes signify? Students shared 1007 documents in this course. Web the relative atomic mass (ar) of atoms is the average mass of all the different isotopes of an element (taking into account the amount of each isotope) on a scale where 12c atoms have a mass of exactly 12. The average atomic mass of a lead atom is 207.2 amu.
32 Protons, 38 Neutrons, 32 Electrons.
What does the number next to isotopes signify? Consider the following illustrations in which protons are shown as pink spheres and neutrons are shown as green spheres. How many protons and neutrons are in the first isotope? Calculate the average atomic mass of chlorine if its isotopes and % abundances are as follows.
The number 6 refers to the _________________________ c. 5 protons, 6 neutrons, 5 electrons. A short worksheet to introduce or revise isotopes. Here are three isotopes of an element: How can you tell isotopes apart?