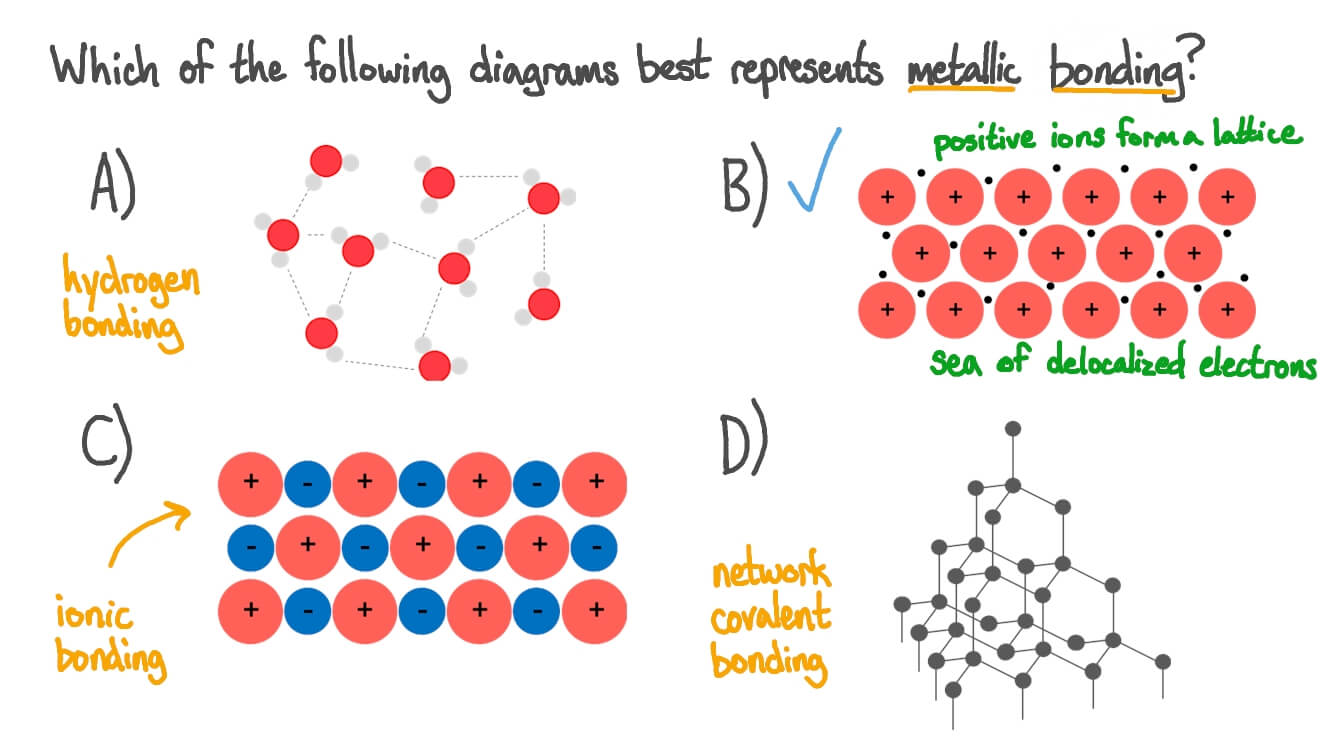
The arrangement of the atoms in a metal. Ionic bonds, covalent bonds and metallic bonds are examples of chemical bonds. We prioritise the core academic subjects that are strong preparation for further study, understanding of the world and fulfilling lives. Metals are said to be giant structures since they usually contain lots of atoms. Web metallic bonds are strong and are a result of the attraction between the positive metal ions and the negatively charged delocalised electrons.
It can be described as the sharing of free electrons among a lattice of positively charged metal ions. Or spread the cost over 60 months for less than £34 per month* highest quality. Metallic lattices do not contain fixed. It creates a bulk of metal atoms, all clumped together.
When there are many of these cations, there are also lots of electrons. How to draw wembley fraggle from. 1.4.7 properties of ionic compounds;
When there are many of these cations, there are also lots of electrons. Describe how the electrical and thermal conductivity of metals can be explained according to band theory. Is the attraction between the positive ions in a regular lattice and the. Is the attraction between the positive ions in a regular lattice and the. The extra electrons on the outer shell leave the atom, making the metal a positive ion.
By emma owens 19 june 2023. Metals tend to form cations. Web a metallic bond is a type of chemical bond in which a ‘cloud’ of free moving valence electrons is bonded to the positively charged ions in a metal.
In Metallic Bonding, Metals Become Cations And Release Out Electrons In The Open.
Metallic bonds are strong, so metals can maintain a regular structure. It can be described as the sharing of free electrons among a lattice of positively charged metal ions. Web a metallic bond is a type of chemical bond in which a ‘cloud’ of free moving valence electrons is bonded to the positively charged ions in a metal. Everything you need to teach metallic bonding.
By Emma Owens 19 June 2023.
The structure and bonding in a substance are. Is the attraction between the positive ions in a regular lattice and the. Web metallic bonds are strong and are a result of the attraction between the positive metal ions and the negatively charged delocalised electrons. Even a metal like sodium (melting point 97.8°c) melts at a considerably higher temperature than the element (neon) which precedes it in the periodic table.
When There Are Many Of These Cations, There Are Also Lots Of Electrons.
Of attraction between the metal ions and the delocalised electrons. The structure of metallic bonds is entirely different from that of ionic and covalent bonds. Delocalised electrons are free to move throughout. Metals tend to form cations.
Web It's Like Ionic Bonding But With A Sea Of Electrons.
We prioritise the core academic subjects that are strong preparation for further study, understanding of the world and fulfilling lives. Metallic bonding in transition elements. Delocalised electrons are free to. Metallic bonding forms between metals and metals.
Metallic bonding forms between metals and metals. Delocalised electrons are free to. Metallic bonding is the strong. Or spread the cost over 60 months for less than £34 per month* highest quality. Everything you need to teach metallic bonding.