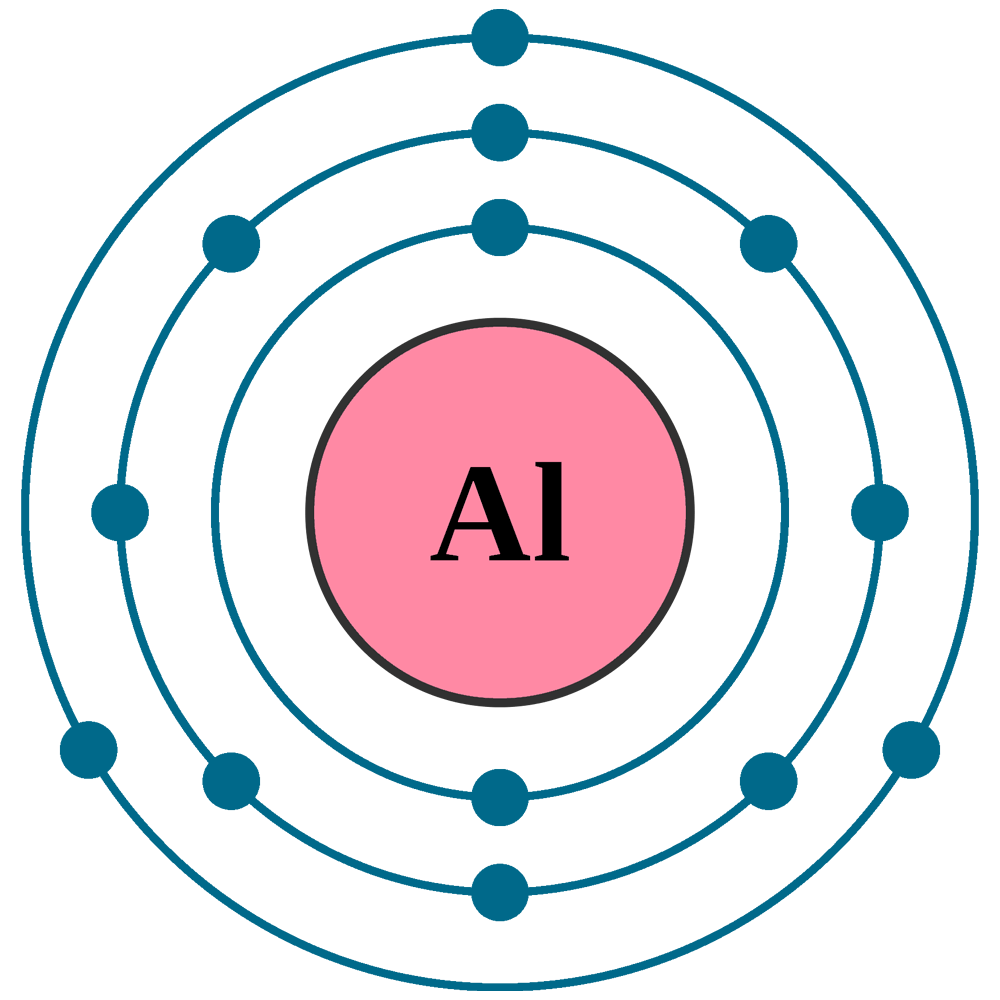
Web aluminum ion(al 3+) electron configuration. To obtain a full outer shell: A cation (positively charged ion) forms when one or more electrons are removed from a parent atom. Predict which forms an anion, which forms a cation, and the charges of each ion. Accordingly, the combined first three ionization energies of aluminium are far lower than the fourth ionization energy alone.
Web because the number of protons remains unchanged when an atom forms an ion, the atomic number of the element must be 13. 31k views 3 years ago. For main group elements, the electrons that were added last are the first electrons removed. Web chemistry for allied health (soult) 2:
Web ions are formed when atoms gain or lose electrons. 18 × ( 1 −) = 18 − net charge: As always, there are exceptions.
Individual atoms can gain or lose electrons. If we want to summarize the process of chlorine gaining an electron to form the ion, we may write it like this: As mentioned above, there are positive and negative ions. Web ions are formed when atoms gain or lose electrons. Al will form a cation with a charge of 3+:
In this video we’ll use the periodic table and a few simple rules to find the number of protons and electrons for the. Write the symbol for each ion and name them. The following table shows how we name the ions for the first four elements in this group:
In This Video We’ll Use The Periodic Table And A Few Simple Rules To Find The Number Of Protons And Electrons For The.
Web remember that ions are formed only when electrons move from one atom to another; Web an aluminium atom has 13 electrons, arranged in an electron configuration of [ ne ] 3s2 3p1, [16] with three electrons beyond a stable noble gas configuration. The following table shows how we name the ions for the first four elements in this group: You then split the electrons between the different orbitals.
Since Aluminum's Atomic Number Is Thirteen, It Has Thirteen Electrons.
A cation (positively charged ion) forms when one or more electrons are removed from a parent atom. Web aluminum ion(al 3+) electron configuration. Electronegativity (pauling scale) the tendency of an atom to attract electrons towards itself, expressed on a relative scale. ( 20 +) + ( 18 −) = 2 +.
The Al Atom Has Lost Three Electrons And Thus Has Three More Positive Charges (13) Than It Has Electrons (10).
This is the aluminum cation. Web chemistry for allied health (soult) 2: Define ion, cation, and anion. A proton never moves from one atom to another.
Predicting Charges On Monatomic Cations And Anions.
A (in anion) becomes before c (cation) in the alphabet. Define the two types of ions. Web to find the electron configuration of an atom, you first need to know the number of electrons that it has. For example, the alkali metals in group 1 on the periodic table tend to form cations with a 1 + charge.
Therefore, an aluminum atom has fourteen neutrons. Define the two types of ions. Click here to learn more! After the electron configuration, the last shell of the aluminum atom has three electrons. The following table shows how we name the ions for the first four elements in this group: